Lateral flow tests (LFTs), also known as lateral flow immunoassays (LFIAs), are essential tools in modern diagnostics, celebrated for their speed, portability, and ease of use. These simple yet powerful devices have gained public recognition during the COVID-19 pandemic and have long been used for pregnancy testing. But their potential goes far beyond these two applications.
In this article, we introduce the principles, key components, terminology, formats, advantages and limitations, and most importantly, the diverse real-world applications of Lateral Flow Tests across healthcare and other industries.
What is a Lateral Flow Test (LFT)?
A lateral flow test is an in vitro diagnostic device (IVD) , considered as rapid diagnostic test, designed to rapidly detect the presence of a specific analyte, such as an antigen, antibody, hormone, or chemical compound, in a liquid sample (e.g. blood, urine, saliva). The detection relies on specific molecular interactions (typically antibody-antigen binding) and the capillary movement of the sample along a porous strip, resulting in a visible signal within minutes.
These tests are a subclass of immunoassays that function without the need for laboratory equipment. Their versatility allows them to serve various purposes and industries, for instance:
- Detection of chronic diseases: Lateral Flow Tests can identify biomarkers linked to specific chronic conditions, such as glucose levels for monitoring diabetes or cardiac markers for diagnosing heart disease.
- Detection of pregnancy: Lateral Flow Tests are commonly used to confirm pregnancy by detecting hormones like hCG in a woman’s urine or blood.
- Detection of drugs: Lateral Flow Tests can identify drugs or their metabolites in various biological samples, making them useful tools for law enforcement and workplace testing.
- Detection of environmental contaminants: Lateral Flow Tests help monitor environmental safety by detecting chemical or biological substances, such as pesticides in soil or water and toxins in food processing environments.
The many names of Lateral Flow Tests
In news articles, scientific papers, and other literature, the variety of terms used to describe lateral flow tests (LFTs) can be confusing, as many rely on broad or non-specific language. While « Lateral Flow Test » remains the most accurate and straightforward term, numerous alternatives, some accurate, others misleading, are commonly used. Understanding the correct terminology is essential to avoid confusion and ensure clear communication.
❌ Inaccurate or overly generic terms
- Rapid test
- Rapid Diagnostic Test (RDT)
- Rapid Antigen Test or Antigen Rapid Test (RAT or ART)
- Rapid Antibody Test (RAT or ART)
- Antigen test
- Antibody test
- Immunoassay test
These names describe either the speed or target of the test but omit the underlying technology, potentially grouping Lateral Flow Tests with other platforms like ELISA or latex agglutination.
✅ Technically accurate terms
- Lateral Flow Test (LFT)
- Lateral Flow Assay (LFA)
- Lateral Flow Immunochromatographic Assay or Lateral Flow ImmunoAssay (LFIA)
- Immunochromatographic Test or Lateral Flow Immunochromatographic Test (ICT)
- Lateral Flow Device (LFD)
- Immunochromatographic lateral flow strip test
- Immunochromatographic test strip
- Rapid Immunochromatographic Test or Immunochromatographic Rapid Test (RIT or IRT)
All these names refer to the same core principle: a capillary-driven immunoassay with chromatographic flow.
Core components of a Lateral Flow Device
A typical LFT consists of several integrated parts working together to produce a rapid result:
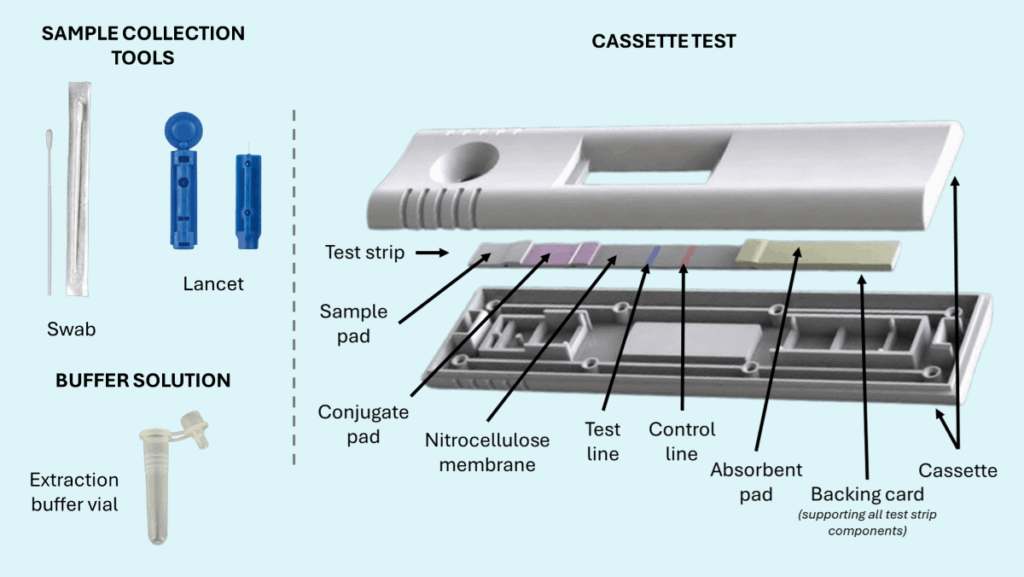
- Test Strip: This is the core of the Lateral Flow Test and consists of several flexible components assembled together.
- Sample Pad: This is the first point of contact for the sample on the cassette. Its function is to condition the sample for optimal flow and binding interactions. It can filter large particles, adjust the pH, or lyse certain molecules to improve sample fluidity.
- Conjugate Pad: This pad contains labeled antibodies or antigens, typically tagged with colored particles such as colloidal gold or latex microspheres, often called detector particles. These particles bind to the target analyte to form the analyte-detector complex and contribute to the visual detection of the result.
- Nitrocellulose Membrane: This is the critical reaction zone and contains at least two detection lines:
- Test line (T): This line features immobilized antibodies or antigens, commonly referred to as capture particles, that bind to the analyte-detector complex migrating from the conjugate pad. A visible line indicates a positive result.
- Control line (C): This line also contains immobilized antibodies or antigens designed to bind detector particles, ensuring the test is working correctly. A visible control line confirms proper fluid migration and test validity.
- Absorbent Pad (or Wick Pad): This pad draws the sample across the nitrocellulose membrane, helping maintain consistent fluid flow.
- Backing Card: This layer provides structural support to the test strip components, enhancing durability and reproducibility during manufacturing.
- Cassette or Housing: This encases the test strip, allowing only the relevant portion of the strip to be visible for result interpretation. It improves handling and usability for the end user.
- Buffer Solution: This optional solution is used to pretreat the raw sample collected from the patient, optimizing its compatibility with the test by adjusting factors like pH or viscosity. In some cases, the sample pad may be pre-treated to serve the same function, making a separate buffer unnecessary.
- Sample Collection Tools: These tools help users collect and apply the sample to the buffer vial or directly onto the sample pad. They may include swabs, lancets, droppers, small vials, or other specialized tools designed for sample collection and transfer.
How do Lateral Flow Tests work?
🧩 Two main assay formats
- Sandwich assays: These are typically used for detecting large molecules that have multiple binding sites, such as proteins. In this format, the target analyte is « sandwiched » between two specific antibodies, one attached to the test line (capture antibody) and the other labeled with a detector (conjugate antibody), allowing for a visible signal if the analyte is present.
- Competitive assays: These are commonly used for small analytes that have only a single binding site or epitope. In this format, a visible test line actually indicates a negative result, as the labeled analyte competes with the target analyte in the sample for binding. If the target analyte is present in sufficient quantity, it prevents the labeled analyte from binding, resulting in the absence of a visible test line.
➡️ Step-by-Step process
1. Applying the sample
The process begins by adding a small volume of liquid, either the raw sample or a pretreated version, into the sample well. Typically, just a few drops are needed. The sample is quickly absorbed by the sample pad located directly beneath the well. This setup ensures the fluid flows perpendicularly through the pad. The sample pad initiates fluid movement across the strip by capillary action. It’s essential to avoid adding too much liquid, as excess volume can flood the strip and compromise test performance.
2. Releasing the conjugates
As the sample continues to flow by capillary action, it reaches the conjugate pad, this is a critical phase of the test:
- In sandwich assays: At the conjugate pad, if the sample contains the target molecule (the analyte), it binds to specific detector molecules pre-deposited on the pad. These detectors are usually antibodies conjugated to visible labels such as colloidal gold or latex particles. The interaction is precisely designed: if detecting an antigen, the labeled antibody binds to one epitope, leaving another site free to interact with the capture line later. For antibody detection, the conjugate may target the constant (Fc) region of the antibody to preserve its ability to bind at the test line.
- In competitive assays: Here, the sample also mixes with labeled detector molecules, typically antibodies. If the target analyte is present, it binds to these antibodies, preventing them from interacting with the immobilized analyte further along the strip. If no analyte is present, the labeled antibodies remain free to bind at the test line.
3. Migration through the membrane
The fluid, now carrying the analyte-detector complex (or unbound conjugates), continues to migrate along a nitrocellulose membrane, the central zone of detection. This membrane includes both the test line and control line:
- In sandwich assays: The analyte-detector complex will bind at the test line if the target analyte is present. The test line contains immobilized capture antibodies (or antigens) that recognize another binding site on the analyte, completing the « sandwich ». A visible line forms here to indicate a positive result.
- In competitive assays: The test line contains immobilized analyte or a structural analog. If the sample contains no analyte, the labeled antibodies will bind here, forming a visible line. If the analyte is present in the sample, it will have already bound the labeled antibodies at the conjugate pad, preventing them from binding at the test line, thus no line appears.
4. Verification at the control line
After passing the test line, the fluid reaches the control line, which serves as an internal procedural check. This line captures any remaining conjugate particles through a different binding mechanism, confirming that the test has functioned correctly and that liquid has flowed properly. A visible control line must always appear for the test result to be valid, regardless of whether the analyte is present.
5. Final absorption
Once it has passed both lines, the remaining fluid is drawn into the absorbent (or wick) pad at the end of the strip. This pad maintains the capillary flow by pulling liquid forward and helps prevent backflow or accumulation of fluid on the membrane, which could interfere with accurate detection.
6. Reading the result
After waiting the recommended duration (usually between 10 and 30 minutes), the result can be interpreted. Most lateral flow tests use colorimetric detection, where visible lines indicate the presence or absence of the analyte. More advanced versions may use fluorescent, electrochemical, or magnetic signals, which might require a dedicated reader. The type of detection label used determines whether the result is visible to the naked eye or needs instrumentation.
- In sandwich assays: A visible line at both the test and control lines indicates a positive result. A line only at the control region indicates a negative result.
- In competitive assays: A visible test line indicates a negative result (no analyte in the sample). If the test line is weak or absent, this suggests the analyte is present and has blocked binding at the test line, indicating a positive result.
Reading and interpreting Lateral Flow Test results
Reading the results of a lateral flow test (LFT) requires careful attention to timing, test format, and visual interpretation. Most Lateral Flow Tests are designed to deliver results within 10 to 30 minutes, and it’s essential to read them strictly within this window. Reading the test too early may result in faint or incomplete lines, while reading it too late can cause fading, diffusion, or changes in line intensity, leading to misleading or invalid results. This time sensitivity is crucial to ensure the reliability and accuracy of the outcome.
In some cases, Lateral Flow Tests are used with enriched samples, such as bacterial cultures incubated for 24 to 48 hours. Although the migration through the strip still takes the usual 10 to 30 minutes, these tests lose one of their key features, rapidity, due to the extended sample preparation time. These are exceptions where the Lateral Flow Test itself remains fast, but the overall diagnostic process is not.
A fundamental feature of every Lateral Flow Test is the control line. This line must always appear to confirm that the test has functioned correctly. Its presence verifies that the sample has migrated properly through the strip and that the reagents have been activated and distributed as intended. If the control line does not appear, the result is invalid, and the test must be repeated with a new device.
As discussed previously, the meaning of the test line depends entirely on the type of assay used, either sandwich or competitive:
- In a sandwich assay, a visible test line indicates a positive result. This means the target analyte is present and has been captured between two antibodies, one in the conjugate pad and the other at the test line. This is the most common format, especially in at-home and public health testing.
- In a competitive assay, the logic is reversed: a test line appears only when the target analyte is absent. If the analyte is present, it binds to the labeled antibody in the conjugate pad and prevents binding at the test line, resulting in no visible line. Misunderstanding which type of assay is being used can easily lead to incorrect interpretation, so it is essential to follow the test’s instructions carefully.
In clinical or research environments, Lateral Flow Test results may be read using digital readers or smartphone-based tools. These devices improve interpretation by capturing images of the strip and quantifying line intensities. This is especially useful for faint lines, providing semi-quantitative or even fully quantitative results. While benchtop or handheld readers are typically used in laboratory settings due to their complexity and cost, smartphone-based readers are increasingly being explored as affordable, accessible alternatives. Although still in development and not widely available commercially, they are expected to grow in relevance, particularly in telemedicine and remote diagnostics.
Some Lateral Flow Tests are capable of detecting multiple targets simultaneously. This can be achieved in two main ways:
- A single strip with one control line and multiple test lines, each targeting a different analyte.
- An array of parallel strips, where each strip targets a separate analyte and has its own control and test lines.
More sophisticated formats may use multiple labels, such as fluorescent dyes with distinct emission wavelengths, allowing for multiplex detection within a single test region. However, these advanced formats require specialized readers and cannot be interpreted visually without instruments.
In certain applications, the goal isn’t to identify specific analytes, but rather to detect a broader group. This is often done using cross-reactive antibodies that recognize multiple related molecules—for example, different strains of the same virus or various antigens from a single pathogen. In such cases, the result appears as a single, undifferentiated signal that confirms at least one target from the group is present, without indicating exactly which one. This approach is especially valuable in screening scenarios, where detecting the group is more important than identifying its exact components.
Different physical formats of Lateral Flow Tests
Lateral Flow Tests are available in various physical formats to suit different applications:
- Cassette format: The most common for all kind of infectious diseases diagnostic, and often used in self-tests.
- Dipstick format: A simple strip dipped directly into a sample.
- Midstream format: Common in pregnancy tests, designed for direct urine application.
- Panel or multiplex tests: Include multiple test strips or detection zones for simultaneous analysis.
- Cup tests: Often used for drug testing, integrating sample collection (urine or feces) and analysis.
- Strip arrays: Multiple targets assessed in parallel with independent strips.
- Electronic cassettes: Encased tests that integrate sensors and offer digital readouts.
What are the strengths and limitations of Lateral Flow Tests (LFTs) ?
💎Advantages
Lateral flow tests offer a range of significant benefits that make them particularly suitable for rapid, point-of-care (POC) diagnostics:
- Rapid turnaround: Results are typically available within 10 to 30 minutes, enabling immediate decision-making.
- Cost-effective and accessible: Lateral Flow Tests are relatively inexpensive to manufacture and use, making them widely available, including in low-resource settings.
- Usable outside laboratories: Their simple design allows for deployment in the field, at home, or in clinics without the need for advanced infrastructure.
- Long shelf life: Most Lateral Flow Tests are stable for extended periods at room temperature, eliminating the need for refrigeration and easing distribution logistics.
- Minimal sample volume: They require only small amounts of biological material, such as a drop of blood, saliva, or a nasal swab extract.
- No specialized equipment needed: Most Lateral Flow Tests are visually read and do not require any instrumentation, making them ideal for decentralized testing.
- Compatibility with digital readers: When paired with optical or smartphone-based readers, Lateral Flow Tests can deliver enhanced precision, traceability, and integration into digital health systems.
- Multiplexing capability: Some Lateral Flow Tests can detect multiple analytes simultaneously, either through multiple test lines or advanced detection technologies, improving diagnostic breadth.
⚠️ Limitations
Despite their many advantages, Lateral Flow Tests do present some challenges and limitations that must be considered:
- Lower sensitivity and specificity: Compared to molecular diagnostics (such as PCR), Lateral Flow Tests generally provide less accurate results, particularly for low-concentration analytes.
- Sample-related interference: Viscous, particulate, or contaminated samples can hinder capillary flow and lead to invalid or unclear results.
- Strict timing requirements: Accurate interpretation depends on reading results within a specific time window; deviations can lead to false positives or negatives.
- Primarily qualitative: Most Lateral Flow Tests provide simple yes/no results rather than quantitative measurements, which may not be sufficient for all clinical or research purposes.
- Environmental concerns: Used Lateral Flow Tests often contain biological materials and chemical reagents, creating challenges for safe disposal, especially at scale.
- Instrument compatibility adds complexity: While readers can improve accuracy, they introduce additional cost, require power sources, and may complicate use in the field.
- Reproducibility and cross-reactivity issues: Variability between lots or improper test design can affect consistency and lead to false results due to non-specific binding.
Examples of applications across fields
Lateral Flow Tests are highly adaptable tools, widely applied across diverse sectors thanks to their portability, speed, and ease of use. Below are some key domains where Lateral Flow Tests have demonstrated their versatility.
🧬 Human health diagnostics
Lateral Flow Tests are routinely used to detect infections caused by viruses, bacteria, fungi, or protozoa by identifying specific antigens or antibodies in bodily fluids such as blood, saliva, or nasal secretions. A notable example is the widespread use of COVID-19 antigen tests during the pandemic. These tests provided results in under 20 minutes without the need for laboratory infrastructure, making them ideal for home use, mass screening, and deployment in resource-limited settings.
🤰 Pregnancy monitoring
One of the most well-known applications of Lateral Flow Tests is in home pregnancy tests. These detect the presence of human chorionic gonadotropin (hCG), a hormone produced shortly after fertilization, in urine. They offer fast, discreet, and reliable results, empowering individuals to monitor early pregnancy in the privacy of their homes.
🩸 Diabetes monitoring
Lateral Flow Test strips can measure glucose or ketone levels in urine or blood, supporting metabolic assessments and flagging conditions such as diabetic ketoacidosis. While they do not replace continuous glucose monitoring systems, these tests serve as quick, accessible tools, especially in emergencies or in locations with limited access to more sophisticated technologies.
🧪 Oncology
Emerging applications in cancer diagnostics include Lateral Flow Tests that detect biomarkers like prostate-specific antigen (PSA), alpha-fetoprotein (AFP), and carcinoembryonic antigen (CEA). These tests offer non-invasive, rapid tools to assist in early detection, monitoring disease progression, or evaluating response to treatment, particularly when used in conjunction with conventional laboratory diagnostics.
💊 Drug testing
Lateral Flow Tests can detect a variety of substances, including recreational drugs like THC, cocaine, and opioids, as well as prescription medications. They are widely used in workplace testing, roadside law enforcement, emergency rooms, and rehabilitation programs. Their rapid turnaround and capacity to detect multiple drug classes in a single test make them indispensable for real-time decision-making.
🐾 Veterinary diagnostics
In animal health, Lateral Flow Tests are used to diagnose infectious diseases such as feline leukemia, canine parvovirus, and livestock pathogens like foot-and-mouth disease. Their portability enables point-of-care use in clinics, farms, and field conditions, aiding in timely intervention and containment.
🥗 Food safety
Lateral Flow Tests play a critical role in the food industry by detecting harmful pathogens (e.g., E. coli, Listeria, Salmonella) and allergens such as peanuts, gluten, or soy. They are used throughout the food production chain to ensure that products meet safety standards before reaching consumers.
🌿 Environmental monitoring
Lateral Flow Tests can detect biological or chemical hazards in environmental samples such as water, air, and soil. Examples include monitoring Legionella in water systems or pesticide residues in agricultural soil. These tests support rapid environmental assessments to protect public health and industrial safety.
🦠 Antimicrobial Resistance (AMR) detection
Specialized Lateral Flow Tests are being developed to identify resistance mechanisms like extended-spectrum beta-lactamases (ESBLs) and carbapenemases. These tests allow clinicians to detect resistant bacterial infections quickly, enabling timely and targeted antibiotic treatment, especially in healthcare settings that lack access to molecular testing platforms.
Conclusion: simple yet powerful diagnostic tools
Lateral flow tests are simple, versatile, and cost-effective diagnostic tools that continue to evolve. From infectious disease screening to environmental monitoring, their robust design and broad applicability make them indispensable across fields.
As the technology advances, with innovations like nanobody-based detection, digital readers, and multiplexing, Lateral Flow Tests are becoming smarter, more sensitive, and more connected. Our company is proud to contribute to this transformation and to provide insight into how these tests work, how to interpret them, and how to best use them in practice.
